Weighted Average Mass of the Mixture of Its Isotopes
Is the weighted average mass of its isotopes. The average atomic mass of an element is the weighted-average mass of the mixture of its isotopes.

Isotopes And Weighted Average Atomic Mass
_____ is the weighted-average mass of an elements isotopes.

. To determine the most abundant isotopic form of an element compare given isotopes to the weighted average on the periodic table. The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit amu also known as daltons D. Positively charged particles in an atom.
Region around the nucleus where the electrons are found. There is a smaller percentage 1 of hydrogen atoms WITH one NEUTRON in their nuclei to give the deuterium isotope. A mixture that is unevenly mixed.
Substance that contains at least 2 elements BONDED together. Calculating Average Atomic Mass Average atomic mass f1M1 f2M2 fnMn where f is the fraction representing the natural abundance of the isotope and M is the mass number weight of the isotope. Individual snack size bags of Chex.
The higher the isotopic percentage the MORE that isotope will contribute to the isotopic mass. Check to make sure that your answer makes sense. Add together the weighted masses to obtain the atomic mass of the element.
The weighted average atomic mass of boron would be calculated. An average of the masses of all the isotopes that occur in nature for an element. Vertical columns on the periodic table.
The atomic mass or weighted average of hydrogen is around 1008 amu look again at the periodic table. The atomic masses of the chemical elements are usually calculated with the weighted average of the masses of the different isotopes of each element taking into account the relative abundance of each of them which explains the non-correspondence between the atomic mass in umas of an element and the number of nucleons that harbors the nucleus of its most. The average atomic mass of an element can be found on the periodic table typically under the elemental symbol.
Weighted average mass of the mixture of its isotopes. The average atomic mass of an element is the weighted-average mass of the mixture of its isotopes. The average atomic mass of the element boron is 108 amu.
Average atomic mass is closest to its most _____ isotope. For example the three hydrogen isotopes shown above are H-1 H-2 and H-3. The quoted atomic mass on the Periodic Table is the WEIGHTED average of the individual isotopic masses.
The atomic weight of an element as shown on the periodic table is the weighted average of the naturally occurring isotopes not all of the isotopes. The mass listed on the periodic table for an element is an average atomic mass or average atomic weight which is an average of the mass numbers of all isotopes of that element weighted by the natural abundance of each isotope. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
Every living organism has isotopes of carbon. The average atomic mass of an element is the weighted-average mass of the mixture of its isotopes. Number of protons and neutrons Subatomic.
Each element exists in nature as a mix of 1 or more isotopessome elements only have 1 natural isotope The mix is reasonably constant so the weighted average of the mix is the CHEMICAL atomic weightmass This is the value shown in the periodic table and can vary a slight amount depending on the source of the element or compound but usually not enough to be concerned. The atomic mass is a weighted average of all of the isotopes of that element in which the mass of each isotope is multiplied by the abundance of that particular isotope. For example boron exists as a mixture that is 199 10 B and 801 11 B.
For example four out of five atoms of boron are boron-11 and one out of five is boron-10. Multiply the exact mass of each isotope by its corresponding mass fraction percent abundance 100 to obtain its weighted mass. The weighted average is determined by multiplying the percent of natural abundance by the actual mass of the isotope.
Particles found within the atom mainly protons neutrons and electrons. 263 07577 34969 u 02423 36966 u 35453 u. A The atomic mass is the weighted average of the masses of the isotopes.
Average Atomic Mass of isotope 1 mass of isotope 1 of isotope 2 mass of isotope 2. The naturally occurring isotopes which give the atomic weight are the stable isotopes and occasionally ones with such a long half-life that they behave as if they were stable. The average atomic mass mass on the periodic table is a weighted average of all isotopes.
Here is a video which summarizes the steps needed to calculate average. To find the weighted-average or the average atomic mass of boron you would solve the following equation. The second method is easier but it doesnt explain intuitively why the answer is the average atomic mass.
Weighted average mass of the mixture of its isotopes. The average mass is the mass of each isotope multiplied by its percentage. For example if we take a weighted average for the isotopes of Carbon we get an average atomic mass of 12011 amu.
The average atomic mass is close to the mass of its most abundant isotope. The Periodic Table and Isotopes Abundance The periodic table only tells us a weighted average of the atomic masses of the different isotopes for an element. The average atomic mass is close to the mass of its most abundant isotope.
Like Carbon many elements exist in nature as a mixture of isotopes. Two particular isotopes Carbon -12 and Carbon -14 are used to determine the age of organisms that may have lived in the past. In this case the average atomic mass of magnesium is 07870 23985 amu 01013 24985 amu 01117 25.
Takes shape and volume of its container. Thus Average mass 5069 7892 u 4931 8092 u 4000 u 3990 u 7991 u. How can you calculate a weighted average mass that represents the average atomic mass of Crunchinumium.
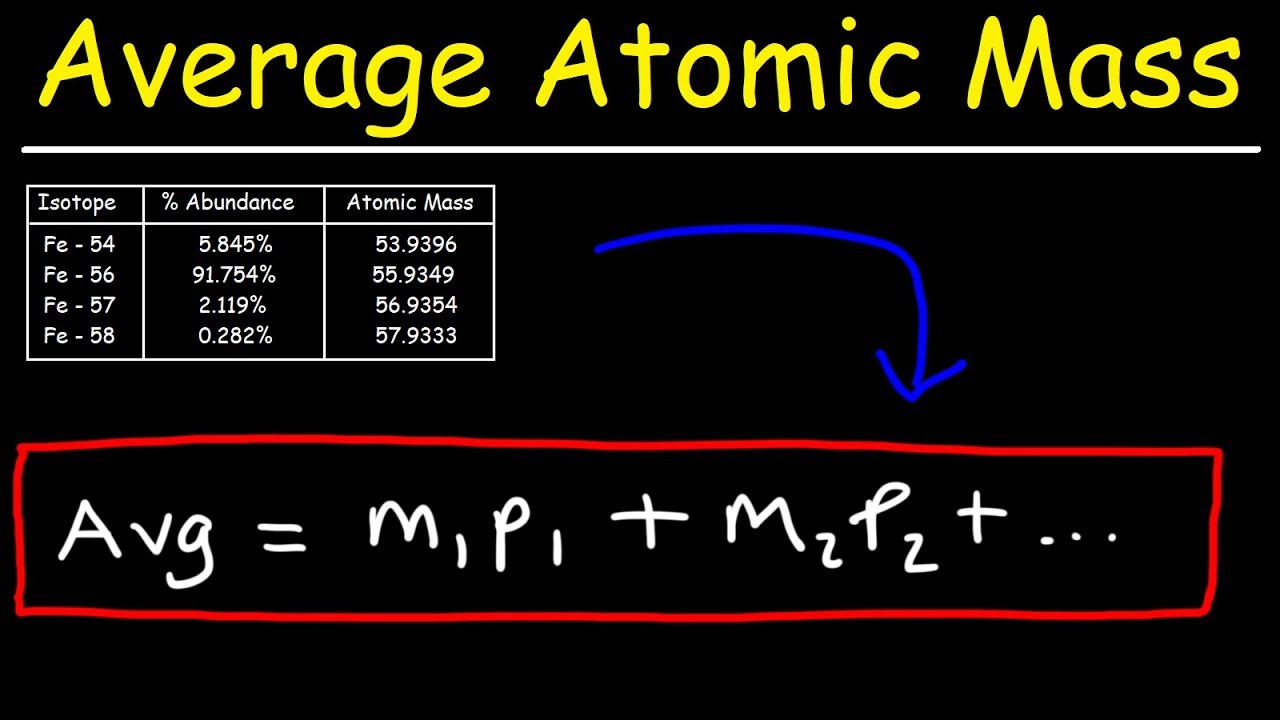
How To Calculate The Average Atomic Mass Youtube
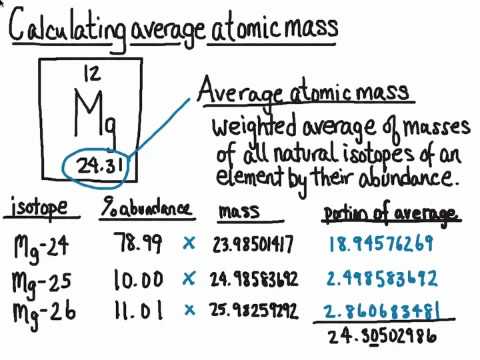
Belum ada Komentar untuk "Weighted Average Mass of the Mixture of Its Isotopes"
Posting Komentar